Ocean Acidification:
How does it work?
The decreasing pH of the oceans, called ocean acidification, is caused by the ocean’s increased uptake of carbon dioxide (CO2). Oceans naturally uptake gases from the atmosphere like CO2. In fact, the oceans absorb about a quarter to a third of human CO2 emissions! That means as our emissions rise, the oceans will take in more and more CO2.
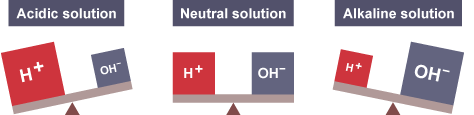
What happens when the ocean absorbs CO2? The gas reacts with water (H2O) and forms carbonic acid. When this acid breaks down it releases Hydrogen ions (H+), which is what makes things like lemon juice acidic. If you have more H+ than hydroxide ions (OH-) then this ratio makes the solution acidic. Examples are vinegar and lemon juice. If there are less H+ than OH- that means the solution is basic, or alkaline. Examples are milk.
Basically, pH is just a scale that measures the ratio of H+ and OH-, or how much of one there is versus the other.
So when we say the oceans are acidifying, what we mean is the oceans are accumulating more and more Hydrogen ions and changing that ratio!
The chemistry of the ocean is changing with the increasing addition of CO2. The addition is throwing a vital process off balance: calcification. This is the process by which shellfish build their shells!
To build their little mobile homes, the shellfish consume something called Calcium carbonate. This molecule forms when calcium and carbonate react to each other and combine. When the two combine they form the perfect building block for shells! But how is this process thrown off by the changing chemistry? This is where all those extra Hydrogen ions come in. The ions react and combine with the carbonate before the calcium can combine with the carbonate!
Shellfish can’t use carbonate when it’s combined with hydrogen. Shellfish can only use the combination of calcium and carbonate to build their shells. As more carbonate is used up by these extra H+ ions, there is less for calcium to combine with, thus there are less shell building blocks available for shellfish to use! Now shellfish must work harder to build the same strength shells with less of the building blocks available to them. This leaves less energy in their reserves to look for food and hide from predators.